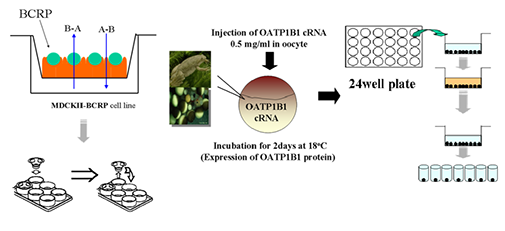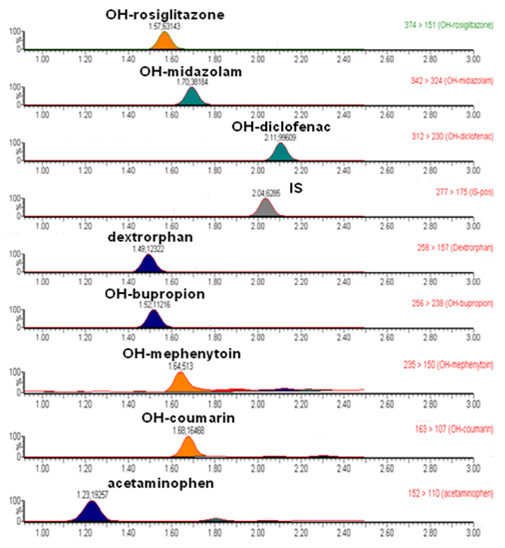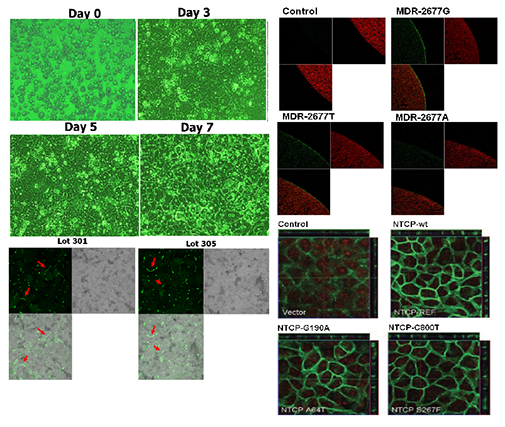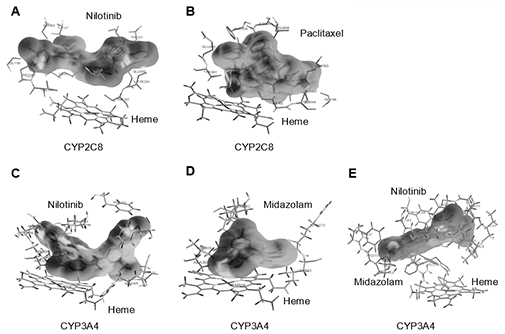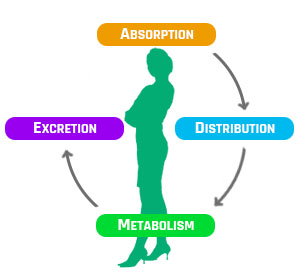
ADME는 “Absorption(흡수), Distribution(분포), Metabolism(대사), Excretion(배설)”의 약자로서,
하나의 약물이 생체 내 목표하는 장기에 이르기까지의 처리되는 과정입니다.
본 검사는 약물의 안전성, 유효성 평가 및 인증에 필요한 ADME 시험항목 서비스를 제공합니다.
| 분류 | 항목 |
|---|---|
| Permeability test | Cell Permeability Test |
| Metabolism study | Metabolic Stability |
| Metabolite Profiling & Identification | |
| Reaction Phenotyping | |
| Transport study | Uptake Transport Study |
| Efflux Transport Study | |
| Cryopreserved Hepatocytes Uptake | |
| Hepatic Excretion by Sandwich Cultured Hepatocytes | |
| Drug-Drug Interaction study | Enzyme Inhibition |
| Time-dependent Inhibition | |
| Transporter Inhibition | |
| Enzyme / Transporter Induction |